
By Kai Kupferschmidt [1,2]
The favorite color of most people, however hardly common among animals and plants. It is very difficult to produce artificially. Consequently, scientists are eagerly working on creating new blue pigments.
Part 1: In the Search of Blue
Can we really be sure where the color blue has its origin? Throughout history, it was a tedious and laborious process – or a stroke of luck.
Pure Coincidence
His most famous discovery came like out of a blue sky. Mas Subramanian, a solid-state chemist at the chemical company Dupont, published hundreds of papers and dozens of patents. He had already discovered a new superconductor and a more environmentally friendly way to produce the chemical fluorobenzene. After joining Oregon State University in 2006, he worked on so-called multiferroics, a material with special electric and magnetic properties, which could lead to faster computers. Based on his idea, the PhD student, Andrew Smith, mixed indium oxide, manganese oxide and yttrium oxide and heated the mixture in an oven. The desired effect did not show, but its color was very blue.BASF spend more money for the synthesis of indigo as it was worth those days
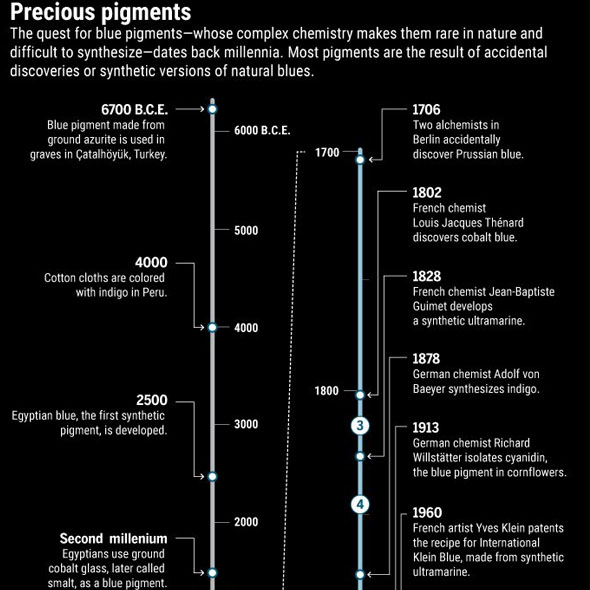
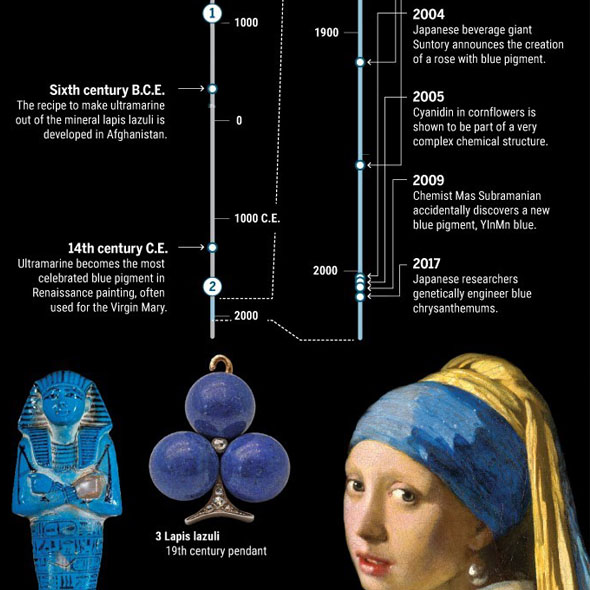
Already 100.000 years ago, pigments as well as charcoal were made of red and yellow ochre, but there was no blue color. The Babylonians and Egyptians used bits of blue lapis lazuli. The tedious process to grind the semi-precious stone into powder and turn into ultramarine was only developed in the 6th century B.C. E. Due to the lack of natural blue, people tried to make this color themselves. Recent discoveries in Turkish gravesites indicate that 9000 years ago the blue mineral azurite was pulverized for cosmetic purposes. 5000 years ago the Egyptians mixed sand, plant ash and copper to produce the first blue synthetic pigment. In the 19th century, chemists were competing to make the first synthetic ultramarine. BASF spend 18 million gold marks – more than the actual company’s value those days - to synthesize indigo, a deep blue dye derived from plants. This kind of blue has become one of the most requested products of the chemical industry.
A pigment or colorant has to absorb red light to appear blue. This occurs when red photons boost electrons in the pigment molecule from one energy level to the next higher energy level. As red light has the lowest energy of any visible light, these two energy levels have to be very close together. Such closely spaced energy rungs are only found in very complex molecules, which is difficult to produce for an organism.
Plants have developed many different pigments. Leaves are green because of the chlorophyll; carotenoids give carrots the orange, tomatoes the red and corn the yellow color; betalain produces the color of red beets. But only one kind of pigment can produce the color blue: the anthocyanin (blue flower). And even most anthocyanins are not blue but red, as they naturally absorb blue light. These molecules have to connect with other chemical groups, only then they are capable to absorb red light.
Even today, it is difficult to design new blue materials from scratch. Subramanian says: “So much chemistry has to come together”. Small changes in the arrangement of neighboring atoms can alter the energy levels of electrons and consequently, the color, which can be absorbed. Ruby red and emerald green originate from chromium ions surrounded by six oxygen atoms. Other atoms in both stones cause the color difference by altering the chromium’s energy levels. Such effects are very hard to predict, Subramanian says: “If rubies and emeralds didn’t exist in nature, nobody would not know how to produce them.”
Nevertheless, scientists have not given up their hunt for the new blue and continue their search with tools of the 21st century. Although his discovery came about by pure accident, other researchers are using physics, chemistry and genetics to find or create new blues.

CharlesJSharp/CC-BY-SA-4.0;
Jan Vermeer/Mauritshuis/The Hague/Bridgeman Images;
© British Library Board/Granger/Two Pence Post Office Mauritius
References:
[1] Das Blaue Wunder, German Newspaper Süddeutsche Zeitung, Weekend Edition, Knowledge Chapter, July 6. / 7, 2019
[2] In Search of Blue, By Kai Kupferschmidt, Science Magazine published by AAAS, May 2nd 2019, https://www.aaas.org/
[3] Blau - Reise durch faszinierende Farbe, Kai Kupferschmidt
https://www.amazon.de/Blau-Reise-durch-faszinierende-Farbe/dp/3455006396
