
By Kai Kupferschmidt [1,2]
The favorite color of most people, however hardly common among animals and plants. It is very difficult to produce artificially. Consequently, scientists are eagerly working on creating new blue pigments.
Part 1: In the Search of Blue
Can we really be sure where the color blue has its origin? Throughout history, it was a tedious and laborious process – or a stroke of luck.
Pure Coincidence
His most famous discovery came like out of a blue sky. Mas Subramanian, a solid-state chemist at the chemical company Dupont, published hundreds of papers and dozens of patents. He had already discovered a new superconductor and a more environmentally friendly way to produce the chemical fluorobenzene. After joining Oregon State University in 2006, he worked on so-called multiferroics, a material with special electric and magnetic properties, which could lead to faster computers. Based on his idea, the PhD student, Andrew Smith, mixed indium oxide, manganese oxide and yttrium oxide and heated the mixture in an oven. The desired effect did not show, but its color was very blue.BASF spend more money for the synthesis of indigo as it was worth those days
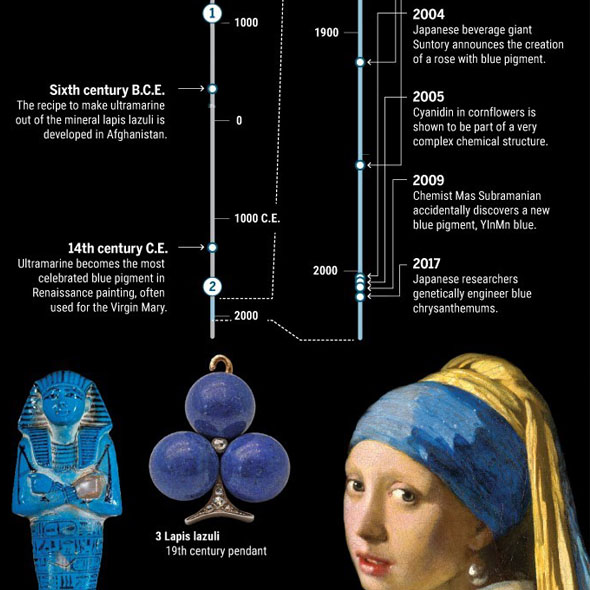
Already 100.000 years ago, pigments as well as charcoal were made of red and yellow ochre, but there was no blue color. The Babylonians and Egyptians used bits of blue lapis lazuli. The tedious process to grind the semi-precious stone into powder and turn into ultramarine was only developed in the 6th century B.C. E. Due to the lack of natural blue, people tried to make this color themselves. Recent discoveries in Turkish gravesites indicate that 9000 years ago the blue mineral azurite was pulverized for cosmetic purposes. 5000 years ago the Egyptians mixed sand, plant ash and copper to produce the first blue synthetic pigment. In the 19th century, chemists were competing to make the first synthetic ultramarine. BASF spend 18 million gold marks – more than the actual company’s value those days - to synthesize indigo, a deep blue dye derived from plants. This kind of blue has become one of the most requested products of the chemical industry.
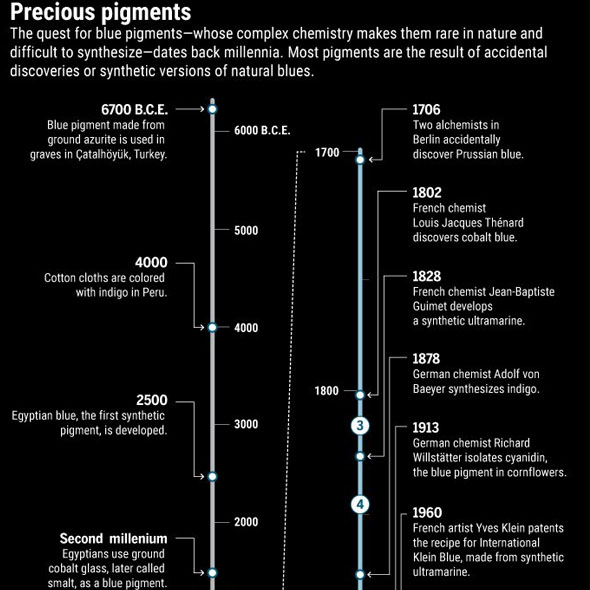
A pigment or colorant has to absorb red light to appear blue. This occurs when red photons boost electrons in the pigment molecule from one energy level to the next higher energy level. As red light has the lowest energy of any visible light, these two energy levels have to be very close together. Such closely spaced energy rungs are only found in very complex molecules, which is difficult to produce for an organism.
Plants have developed many different pigments. Leaves are green because of the chlorophyll; carotenoids give carrots the orange, tomatoes the red and corn the yellow color; betalain produces the color of red beets. But only one kind of pigment can produce the color blue: the anthocyanin (blue flower). And even most anthocyanins are not blue but red, as they naturally absorb blue light. These molecules have to connect with other chemical groups, only then they are capable to absorb red light.
Even today, it is difficult to design new blue materials from scratch. Subramanian says: “So much chemistry has to come together”. Small changes in the arrangement of neighboring atoms can alter the energy levels of electrons and consequently, the color, which can be absorbed. Ruby red and emerald green originate from chromium ions surrounded by six oxygen atoms. Other atoms in both stones cause the color difference by altering the chromium’s energy levels. Such effects are very hard to predict, Subramanian says: “If rubies and emeralds didn’t exist in nature, nobody would not know how to produce them.”
Nevertheless, scientists have not given up their hunt for the new blue and continue their search with tools of the 21st century. Although his discovery came about by pure accident, other researchers are using physics, chemistry and genetics to find or create new blues.

CharlesJSharp/CC-BY-SA-4.0;
Jan Vermeer/Mauritshuis/The Hague/Bridgeman Images;
© British Library Board/Granger/Two Pence Post Office Mauritius
By Kai Kupferschmidt [1,2]
The favorite color of most people, however hardly common among animals and plants. It is very difficult to produce artificially. Consequently, scientists are eagerly working on creating new blue pigments.
Part 2: The Impossible Flower
Since romanticism the blue flower has been a symbol for longing and the unreachable. No matter what has been tried, the perfect blue flower is still far away.
The first blue rose was introduced to the public by the Japanese researcher Yoshikazu Tanaka and his staff in 2004. The only problem: it wasn’t that blue. Although its flower petals produced a blue pigment, the rose itself appeared purple. Tanaka himself admitted that the rose could have been “more blue”.
Still searching for the blue rose Tanaka now is working in the research lab of the beverage company called Suntory, also Japan’s first whiskey distillery. The tax increase in the 1980s caused the company to enter the flower market. Company legend has it that the English Rose of Suntory should have had the Scottish national color blue to celebrate the invention of whiskey.
It turned out to be a smart marketing idea for there are hardly any blue flowers besides artificially ones like blue orchids. Chrysanthemums, carnations, tulips - none of them naturally come in blue. The same is true for blue orchids, which have usually been artificially dyed. Roses are only common in various shades of yellow, pink and red.

Artists have known this fact for a long time. In romanticism, the blue flower was symbol for yearning and the unreachable. Rudyard Kipling wrote a poem about a man who was in search of a blue rose for his beloved: “Half the world I wandered through seeking where such flowers grew.” After his return empty handed his great love has died.
The complexity of the blue blossoms became evident for the first time in 1913. German scientist Richard Willstätter allegedly isolated the blue pigment from cornflowers. It was an anthocyanin, which he named cyanidin. Two years later, he performed the same procedure on red roses and it turned out to be the same molecule. Anthocyanins can change color depending on the acidity of a solution, so Willstätter proposed that roses had a different hue because the pH in their petals was lower than in cornflowers.
This first scientific thesis about blue flowers turned out to be utterly incorrect. It took decades until finally in 2005 an x-ray crystallography confirmed a different statement. A solid blue color is not etablished by cyanidin only. Cornflowers combine six cyanidin molecules with six molecules of a colorless co-pigment, which is arranged around two metal ions – a huge molecule complex that stabilizes the cyanidin molecules and enables the electrons to perform the proper energy transition. “Flowers do weird chemistry to create this kind of blue,” botanist Beverley Glover of Cambridge University in the United Kingdom says.
A few other flowers use the same trick, but most produce a different anthocyanin called delphinidin, which facilitates the blue appearance. It has an additional oxygen atom on one of its rings, which is formed by an enzyme called flavonoid 3’,5’ hydroxylase. The entire family of roses is missing this very enzyme. Thus, delphinidin-producing cannot be created by traditional breeding.
Therefore, Tanaka and his staff counted on genetic engineering. Until 1991, they identified and patented the flavonoid 3’5’-hydroxylase gene in petunias. They planted the gene into carnations and so the production of delphinidin was enforced turning them into a purplish blue. Roses, however, denied the gene. The flower produced delphinidin, to be sure, but no blue pigments.
Tanaka has since tried to cross the genes of various flowers, such as violas with other blue blossoms in order to “decorate” delphinidin chemically, hoping to find the magical combination. Last year he presented hundreds of tiny rose buds which had grown under fluorescent light, a contribution to the development of a new blue color.


Genetic engineering created blue chrysanthemums. Why didn’t it work with roses?
In the meanwhile, a group of Tanaka’s scientists succeeded in creating the one and only blue flower: the blue chrysanthemum. A combination of the flavonoid-3’,5’-hydroxylase gene of the bellflower and a gene that adds a glucose molecule lead to this very success. By means of genetic engineering, the bluest flower the world has ever seen was created. The connection of natural glucose and other pigments with the delphinidin molecule made this happen. This same procedure, however, did not work with roses due to the missing co-pigments and the low pH-value.
However, Tanaka has not given up. He continues experimenting with genes of blue flowers, like from gentian to modify the delphinidin and genes from the genus Torenia that produces co-pigments. In a nod to Willstätter, he is even trying to alter the pH-value of rose blossoms. Tanaka is confident he will develop bluer roses before his retirement, only 5 years away. However, almost thirty years of research also made him more careful: “you never know how blue they will be.”
By Kai Kupferschmidt [1,2]
The favorite color of most people, however hardly common among animals and plants. It is very difficult to produce artificially. Consequently, scientists are eagerly working on creating new blue pigments.
Part 3: Too much Green
The food industry is desperate. How can they be successful finding natural blue ingredients, which are long lasting and resistant to heat as well as light?
A decade ago, Cathie Martin, scientist John-Innes-Center, created a genetic engineered tomato, which produces anthocyanin. This fruit possesses healthy antioxidants and its pigments dye vegetables in a darkish violet blue. Martin therefore had the idea to color other foods blue as well.
A natural blue is not very common among food. In former times, a synthetic ultramarine was used to change the yellowish color of sugarcane into a bright white. Blue food colorings are used for sweets, icings and beverages. They are also used for mixing other colors. “We need blue to produce all colors of the spectrum,” says Richard van Breemen, a chemist at Oregon State University who works with natural products.
The selection is limited at the moment. There are two synthetic food colors approved in the U.S.: brilliant blue also known as Blue No. 1 made out of char tar and Blue No 2, which is gained, from a synthetic indigo blue. Another synthetic blue is available in the EU, called patent blue V. It gives the Curacao Liqueur its blue color, for example.
The consumer, however, prefers natural ingredients. Companies like Mars and Pepsi have been trying to substitute synthetic colors with little success, though. “One of the big disappointments with the color blue is that it is very, very difficult to combine natural colors with compounds that can be used to color food," says Martin. The only natural blue colorant is a raw extract of spirulina algae, which was approved by the U.S. Food and Drug Administration in 2014 for use in confectionery and other foods. However, it is not very stable and blue. “It’s a terrible blue”, she says, “actually it’s green”. And the color might change or even disappear when food is baked, cooked or exposed to light on supermarket shelves.
Van Breemen therefore had been looking for better candidates in the world of microbes. He assumed to find a more stable blue in an extreme environment such as the hot springs in Yellowstone Park or in the ocean. But he could not find any suitable blue pigments. Many substances turned out to be chemical weapons of the microbes. Many substances turned out to be chemical weapons of the microbes. They rather should be used as antibiotics than food colorings.
Plants may be the better choice, especially as they offer a wide range of active ingredients to choose from. Although forming pigments on the basis of delphinine most blue blossoms vary the molecule adding various chemical groups. Cathie Martin hopes to find a stable food coloring in the blue clitoris, a butterfly flower. The beautiful blue flowers give the Malaysian rice dish Nasi Kerabu its colour.
Martin first bought clitoral flowers online at Amazon, but soon stocks ran out. Recently she received three bulging bags of flowers from Saudi Arabia. A scientist who had visited her laboratory had collected these in the wild. A mixture of anthocyanin from the blue clitoris has already proven successful for some food applications, says Martin. Researchers in her lab have used it to make bluish icing for cupcakes and donuts as well as blue ice cream.
But these pigments are also volatile. "Most blue anthocyanin have a half-life of about 24 hours. And we are talking about something that lasts at least three months," says Martin. So their search continues.

A bluish doughnut frosting developed in Cathie Martin's lab contains a mix of anthocyanins found in butterfly pea flowers.
References:
[1] Das Blaue Wunder, German Newspaper Süddeutsche Zeitung, Weekend Edition, Knowledge Chapter, July 6. / 7, 2019
[2] In Search of Blue, By Kai Kupferschmidt, Science Magazine published by AAAS, May 2nd 2019, https://www.aaas.org/
[3] Blau - Reise durch faszinierende Farbe, Kai Kupferschmidt,
https://www.amazon.de/Blau-Reise-durch-faszinierende-Farbe/dp/3455006396



